Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kumagai, Yuta
Hoshasen Kagaku (Internet), (115), p.43 - 49, 2023/04
Oxidation and dissolution of uranium oxide materials has been a subject of numerous studies as a basis of the geological disposal technology for spent nuclear fuel. The understandings obtained by these studies provide useful suggestions for research and development regarding the retrieval and storage of nuclear fuel debris generated by a nuclear severe accident. Here, these research backgrounds of oxidative dissolution of uranium oxides are briefly reviewed and some studies relating to radiation-induced reactions will be introduced.
 )O
)O (
( = Th, Np) prepared by supercritical hydrothermal synthesis
= Th, Np) prepared by supercritical hydrothermal synthesisShirasaki, Kenji*; Tabata, Chihiro*; Sunaga, Ayaki*; Sakai, Hironori; Li, D.*; Konaka, Mariko*; Yamamura, Tomoo*
Journal of Nuclear Materials, 563, p.153608_1 - 153608_11, 2022/05
Times Cited Count:2 Percentile:53.91(Materials Science, Multidisciplinary)We focused on the direct synthesis of (U,  )O
)O solid solution (
solid solution ( =Th, Np) by extending our recent progress in hydrothermal synthesis with additives. The homogeneity of the (U,
=Th, Np) by extending our recent progress in hydrothermal synthesis with additives. The homogeneity of the (U,  )O
)O (
( = Th, Np) systems prepared by supercritical hydrothermal reactions was investigated through crystallographic analysis based on Vegard's law, and the
= Th, Np) systems prepared by supercritical hydrothermal reactions was investigated through crystallographic analysis based on Vegard's law, and the 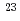 Na nuclear magnetic resonance (NMR) measurement of (U, Np, Na)O
Na nuclear magnetic resonance (NMR) measurement of (U, Np, Na)O solid solutions. Our experimental and analytical results revealed that (i) an optimal additive is ammonium carbonate and starting uranium valence is IV in the case of (U, Th)O
solid solutions. Our experimental and analytical results revealed that (i) an optimal additive is ammonium carbonate and starting uranium valence is IV in the case of (U, Th)O , and (ii) an optimal additive is ethanol and starting uranium valence is VI in the case of (U, Np)O
, and (ii) an optimal additive is ethanol and starting uranium valence is VI in the case of (U, Np)O , for producing the homogeneous solid solutions by hydrothermal synthesis.
, for producing the homogeneous solid solutions by hydrothermal synthesis.
 ; Stoichiometry, crystal shape and size, and homogeneity observed using
; Stoichiometry, crystal shape and size, and homogeneity observed using 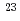 Na-NMR spectroscopy of (U, Na)O
Na-NMR spectroscopy of (U, Na)O
Tabata, Chihiro*; Shirasaki, Kenji*; Sunaga, Ayaki*; Sakai, Hironori; Li, D.*; Konaka, Mariko*; Yamamura, Tomoo*
CrystEngComm (Internet), 23(48), p.8660 - 8672, 2021/12
Times Cited Count:5 Percentile:64.74(Chemistry, Multidisciplinary)The hydrothermal synthesis of pure uranium dioxide under supercritical water (SCW) conditions was investigated. The nonstoichiometry, crystallite size and morphology of the UO particles were investigated. The SCW hydrothermal synthesis may be a promising method for producing homogeneous UO
particles were investigated. The SCW hydrothermal synthesis may be a promising method for producing homogeneous UO and its solid solutions with well-defined nonstoichiometries (
and its solid solutions with well-defined nonstoichiometries ( ), shapes, and sizes.
), shapes, and sizes.
 Ln
Ln O
O (Ln = Gd, Er) solid solution
(Ln = Gd, Er) solid solutionPham, V. M.*; Arima, Tatsumi*; Inagaki, Yaohiro*; Idemitsu, Kazuya*; Akiyama, Daisuke*; Nagai, Takayuki; Okamoto, Yoshihiro
Journal of Nuclear Materials, 556, p.153189_1 - 153189_9, 2021/12
Times Cited Count:1 Percentile:16.35(Materials Science, Multidisciplinary)The crystal structure change was evaluated for (1-x)UO -xLnO
-xLnO (Ln=Gd or Er; x = 0 to 0.4) samples sintered at 1973 K for 8 h under Ar and Ar-10%H
(Ln=Gd or Er; x = 0 to 0.4) samples sintered at 1973 K for 8 h under Ar and Ar-10%H atmospheres. The effect of LnO
atmospheres. The effect of LnO doping on the crystal structure of UO
doping on the crystal structure of UO was investigated by X-ray diffraction (XRD) and X-ray absorption fine structure (XAFS). LnO
was investigated by X-ray diffraction (XRD) and X-ray absorption fine structure (XAFS). LnO doping into UO
doping into UO reduced the lattice parameter of UO
reduced the lattice parameter of UO -LnO
-LnO solid solutions up to 40mol% LnO
solid solutions up to 40mol% LnO . The lattice parameters of these samples were comparable to those of stoichiometric (U,Ln)O
. The lattice parameters of these samples were comparable to those of stoichiometric (U,Ln)O solid solutions, that is, the O/M ratios were close to 2.00. The U L
solid solutions, that is, the O/M ratios were close to 2.00. The U L -edge XANES analysis showed that higher U oxidation states of +5 or +6 formed, in addition to + 4. The EXAFS analysis indicated that the interatomic distances of U-O and Gd-O decreased with increasing x, whereas those of Er-O may not decrease monotonically.
-edge XANES analysis showed that higher U oxidation states of +5 or +6 formed, in addition to + 4. The EXAFS analysis indicated that the interatomic distances of U-O and Gd-O decreased with increasing x, whereas those of Er-O may not decrease monotonically.
Kumagai, Yuta; Takano, Masahide; Watanabe, Masayuki
Journal of Nuclear Materials, 497, p.54 - 59, 2017/12
Times Cited Count:13 Percentile:78.05(Materials Science, Multidisciplinary)We studied oxidative dissolution of uranium and zirconium oxide [(U,Zr)O ] in aqueous H
] in aqueous H O
O solution. The interfacial reaction is essential for anticipating how a (U,Zr)O
solution. The interfacial reaction is essential for anticipating how a (U,Zr)O -based molten fuel may chemically degrade after a severe accident under influence of ionizing radiation. We conducted our experiments with (U,Zr)O
-based molten fuel may chemically degrade after a severe accident under influence of ionizing radiation. We conducted our experiments with (U,Zr)O powder and quantitated the H
powder and quantitated the H O
O reaction via dissolved U and H
reaction via dissolved U and H O
O concentrations. The dissolution yield relative to H
concentrations. The dissolution yield relative to H O
O consumption was far less for (U,Zr)O
consumption was far less for (U,Zr)O compared to that of UO
compared to that of UO . The reaction kinetics indicates that most of the H
. The reaction kinetics indicates that most of the H O
O catalytically decomposed to O
catalytically decomposed to O at the surface of (U,Zr)O
at the surface of (U,Zr)O . We confirmed the H
. We confirmed the H O
O catalytic decomposition via O
catalytic decomposition via O production (quantitative stoichiometric agreement). In addition, post-reaction Raman scattering spectra of the undissolved (U,Zr)O
production (quantitative stoichiometric agreement). In addition, post-reaction Raman scattering spectra of the undissolved (U,Zr)O showed no additional peaks (indicating a lack of secondary phase formation). The (U,Zr)O
showed no additional peaks (indicating a lack of secondary phase formation). The (U,Zr)O matrix is much more stable than UO
matrix is much more stable than UO against H
against H O
O -induced oxidative dissolution.
-induced oxidative dissolution.

Sakai, Hironori; Kato, Harukazu; Tokunaga, Yo; Kambe, Shinsaku; Walstedt, R. E.; Nakamura, Akio; Tateiwa, Naoyuki*; Kobayashi, Tatsuo*
Journal of Physics; Condensed Matter, 15(28), p.S2035 - S2037, 2003/07
Times Cited Count:3 Percentile:21.33(Physics, Condensed Matter)The cubic-fluorite type uranium dioxide UO is an ionically bounded insulator with localized magnetic moments of U
is an ionically bounded insulator with localized magnetic moments of U (5
(5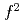 ) ions. Under atmospheric pressure, UO
) ions. Under atmospheric pressure, UO exhibits a first order antiferromagnetic transition at
exhibits a first order antiferromagnetic transition at 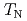 =30.8 K accompanied by a small lattice distortion. In order to clarify the interrelation between QQ and exchange interactions in this system, we performed d.c. magnetization measurement under high pressure up to about 1 GPa by a piston-cylinder-type cramped cell. From the d.c. magnetization measurements under high pressure (
=30.8 K accompanied by a small lattice distortion. In order to clarify the interrelation between QQ and exchange interactions in this system, we performed d.c. magnetization measurement under high pressure up to about 1 GPa by a piston-cylinder-type cramped cell. From the d.c. magnetization measurements under high pressure (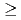 0.1 GPa), it is found that UO
0.1 GPa), it is found that UO shows a weak-ferromagnetic-like transition at
shows a weak-ferromagnetic-like transition at  30 K, where the transition temperature is almost independent of external pressure up to 1 GPa. The ferromagnetic component per uranium atom is very small (
30 K, where the transition temperature is almost independent of external pressure up to 1 GPa. The ferromagnetic component per uranium atom is very small (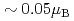 ), although its component is dependent of pressure. This small ferromagnetic component may come from a canted antiferromagnetic ordering.
), although its component is dependent of pressure. This small ferromagnetic component may come from a canted antiferromagnetic ordering.
 fluid leaching (SFL) of uranium from solid wastes using HNO
fluid leaching (SFL) of uranium from solid wastes using HNO -TBP complex as a reactant
-TBP complex as a reactantTomioka, Osamu*; Meguro, Yoshihiro; Iso, Shuichi; Yoshida, Zenko; Enokida, Yoichi*; Yamamoto, Ichiro*
Proceedings of International Solvent Extraction Conference 2002 (CD-ROM), p.1143 - 1147, 2002/00
no abstracts in English
 medium containing HNO
medium containing HNO -TBP complex
-TBP complexTomioka, Osamu*; Meguro, Yoshihiro; Iso, Shuichi; Yoshida, Zenko; Enokida, Yoichi*; Yamamoto, Ichiro*
Journal of Nuclear Science and Technology, 38(6), p.461 - 462, 2001/06
Times Cited Count:27 Percentile:85.72(Nuclear Science & Technology)no abstracts in English
 and BaUO
and BaUO in atmospheres simulating accident conditions
in atmospheres simulating accident conditionsHuang, J.*; Yamawaki, Michio*; Yamaguchi, Kenji*; Yasumoto, Masaru*; Sakurai, Hiroshi*; Suzuki, Yasufumi
Journal of Nuclear Materials, 248, p.257 - 261, 1997/09
no abstracts in English
 and BaUO
and BaUO in atmospheres simulating accident conditions
in atmospheres simulating accident conditionsHuang, J.*; *; Yamaguchi, Kenji*; *; *;
Journal of Nuclear Materials, 248, p.257 - 261, 1997/00
Times Cited Count:6 Percentile:47.96(Materials Science, Multidisciplinary)no abstracts in English
 by means of mass spectrometry
by means of mass spectrometry*; Huang, J.*; Yamaguchi, Kenji*; *; Sakurai, Hiroshi*;
Journal of Nuclear Materials, 231, p.199 - 203, 1996/00
Times Cited Count:11 Percentile:68.11(Materials Science, Multidisciplinary)no abstracts in English
 m class microspheres of ThO
m class microspheres of ThO and (Th,U)O
and (Th,U)O
; Takahashi, Yoshihisa
JAERI-M 93-122, 18 Pages, 1993/06
no abstracts in English
Yamashita, Toshiyuki
JAERI 1310, 84 Pages, 1988/03
no abstracts in English
 -PrO
-PrO

 -O
-O system
system; Fujino, Takeo; Tagawa, Hiroaki
Journal of Nuclear Materials, 132, p.192 - 201, 1985/00
Times Cited Count:21 Percentile:89.56(Materials Science, Multidisciplinary)no abstracts in English
 U
U
 O
O

 solid solution
solid solution; Fujino, Takeo
Journal of Nuclear Materials, 136, p.117 - 123, 1985/00
Times Cited Count:14 Percentile:83.09(Materials Science, Multidisciplinary)no abstracts in English
Nihon Genshiryoku Gakkai-Shi, 27(1), p.15 - 20, 1985/00
Times Cited Count:0 Percentile:0.02(Nuclear Science & Technology)no abstracts in English
Journal of Chemical Physics, 81(12), p.6130 - 6135, 1984/00
Times Cited Count:6 Percentile:29.02(Chemistry, Physical)no abstracts in English
Fujino, Takeo;
Fresenius'Z.Anal.Chem., 314, P. 156, 1983/00
no abstracts in English